Imagine a device that can significantly reduce the risk of stroke for those living with atrial fibrillation (AFib). Enter the WATCHMAN device. This innovative, permanent heart implant provides help for AFib patients by lowering stroke risk and potentially eliminating the need for long-term blood thinners. Let’s dive into how it works, the implant procedure, and its effectiveness in enhancing the quality of life for those diagnosed with AFib.
Short Summary
-
The WATCHMAN Device is an FDA-approved atrial appendage closure device used to reduce stroke risk in AFib patients.
-
It offers a minimally invasive alternative to blood thinning medication, allowing for improved quality of life and reduced anticoagulant use.
-
Clinical trials demonstrate its efficacy with a high success rate and low safety events, making it suitable for many patients living with atrial fibrillation.
Understanding the WATCHMAN Device
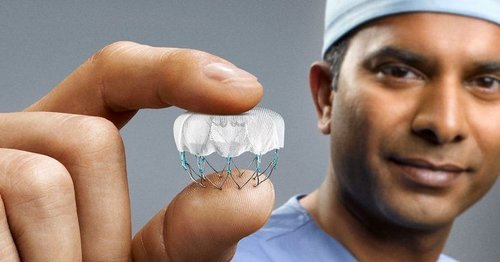
The WATCHMAN Device is a small, permanent implant designed to reduce stroke risk in AFib patients. Atrial fibrillation, the most prevalent type of cardiac arrhythmia, affects the heart’s ability to pump blood, leading to an irregular heartbeat and decreased blood flow. This irregular heartbeat can cause blood clots to form in the heart, increasing the risk of stroke.
The WATCHMAN Device, an atrial appendage closure device, addresses this issue by occluding the left atrial appendage (LAA), the primary source of stroke-causing clots in AFib patients, thus preventing the entry of blood clots into the bloodstream and ensuring that no blood clot escapes. This minimally invasive procedure is an attractive alternative to long-term blood thinners, such as warfarin, eliquis, or xarelto.
The WATCHMAN Device has been successfully implanted in over 100,000 patients worldwide, garnering FDA approval since 2015 and becoming a popular treatment option in more than 50 countries.
The Role of Left Atrial Appendage (LAA)
The left atrial appendage (LAA) is a small pouch in the left atrium of the heart and plays a crucial role in blood clot formation for AFib patients. Around 95% of cases occur due to formation of clots in the LAA (left atrial appendage). Atrial fibrillation can lead to blood pooling in the LAA, which can cause clot formation due to blood cells sticking together. If a clot detaches from the LAA and travels to other parts of the body, it can block blood supply to the brain, resulting in a stroke. Patients with atrial fibrillation are 5x more likely to have a stroke then patients without atrial fibrillation.
The WATCHMAN Device works by occluding the LAA, preventing potentially embolic material from entering the systemic circulation and thus reducing the risk of stroke. By closing off the LAA, the WATCHMAN Device effectively eliminates the primary source of stroke-causing blood clots in AFib patients, and reducing the need for strong blood thinning medication.
Benefits of the WATCHMAN Device
Beyond its ability to reduce stroke risk, the WATCHMAN Device offers several advantages over traditional treatment options like blood thinners. The minimally invasive procedure eliminates or decreases the long term need for strong blood thinners, reducing the risk of heavy bleeding.
This alternative to blood-thinning medication, such as warfarin, eliquis, and xarelto, allows patients to prevent blood clots and enjoy a higher quality of life without worrying about the complications associated with long-term anticoagulant use.
Announced in September 2022, patients who undergo a WATCHMAN implant are no longer required to take any strong anticoagulation medications immediately after the procedure. The device received FDA approval in 09/2022 to use two lighter blood thinners – such as aspirin and clopidogrel – as soon as the procedure is completed. Prior to this announcement, patients still needed to take stronger oral anticoagulation medications, such as warfarin or eliquis, for 45 days after the implantation.
Long-term, patients are able to stop taking strong blood thinning medications, while maintaining the benefits of stroke risk reduction, and reducing the risks of major bleeding. Lighter blood thinners, such as a single low dose aspirin, are still preferable for long term use, even after a watchman implant.
Your Complete Guide To AFib: The Essential Manual For Every Patient With Atrial Fibrillation
$15.95 (as of June 1, 2025 14:05 GMT -06:00 – More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)The Procedure: Implanting the WATCHMAN Device
The implantation of the WATCHMAN Device is a comprehensive process, encompassing preparation, insertion, and recovery. The device is implanted through a minimal incision (usually about 1 cm) in the upper leg and navigated into the LAA of the heart’s upper chambers using a catheter. For the Watchman device, the procedure involves inserting a catheter through a small incision in the right groin and guiding it into the heart while the patient is under general anesthesia.
Once the WATCHMAN Device is in position in the LAA, its frame and mesh covering expand to accommodate the size of the left atrial appendage opening. Several steps are involved to determine if the expanded watchman device is satisfactorily placed in the LAA prior to release of the device from the catheter delivery system. Ultrasound devices are typically used during the procedure to ensure the device is well positioned in the LAA prior to device release from the delivery system. This one-time procedure typically takes around one hour.
Preparing for the Procedure
Before the WATCHMAN Device implantation, patients may undergo various tests and evaluations, such as a transesophageal echocardiogram (TEE) or CT scan, to confirm the absence of clots and to evaluate if the appendage is suitable for one of the available device sizes and shapes.
The procedure is usually performed under general anesthesia, ensuring patient comfort throughout the process.
Recovery and Follow-Up
Following the WATCHMAN Device implantation, patient will usually need to lay in a recovery bed for 2-4 hours post procedure. For my patients, most patients are able to go home the same day of the procedure, although a small number of patients may spend the night in the hospital. The recovery process includes period of limited physical activity. I usually tell patients to take it easy for 3 days, meaning no prolonged standing or walking. After 3 days most normal activities are fine, although I council my patient to refrain from strenuous activities such as heavy lifting or exercise for 1 week. It’s essential for patients to attend follow-up appointments with their physician to ensure proper healing and monitor the device’s effectiveness in reducing stroke risk.
Regular check-ups are important to ensure the device is working properly and to monitor the patient. Typically, some post procedure imaging is performed to ensure positioning and seal of the left atrial appendage, which can be done as early as 6 weeks post procedure or later, this can be performed via a transesophageal echo or a dedicated cardiac CTA.

Effectiveness and Safety of the WATCHMAN Device
FDA Approval and Effectiveness
The WATCHMAN Device’s success in clinical trials led to its FDA approval in 2015 and subsequent global adoption. This innovative treatment is now available in more than 50 countries, offering a safe and effective alternative to long-term blood thinners for AFib patients at high risk of stroke.
Several research studies have been performed to investigate the efficacy of the WATCHMAN procedure. In a 5-year study conducted to compare the WATCHMAN procedure to traditional treatment with warfarin, it was found that the procedure was as effective as anticoagulant treatment for stroke risk reduction. Additionally, patients who had the WATCHMAN procedure performed were less likely to experience significant bleeds and hemorrhages.
In a study done in 2020, researchers explored the complication rate in patients who underwent a WATCHMAN procedure within a two-year period. In the study, 38,158 WATCHMAN procedures were completed across America. In these cases, 98.1% of cases were implanted correctly, and the in-hospital complication rate was 2.16%. Of these complications, the most common complications were pericardial effusion requiring intervention (1.39%) and major bleeding (1.25%), whereas stroke (0.17%) and death (0.19%) were very rare.
Additionally, another study done in 2021 assessed the safety of the newer version of the device, the WATCHMAN FLX LAA closure device. Of the 400 patients in the study, 100% experienced primary effectiveness from the procedure, and only seven patients experienced minor complications afterward. Of these 7 complications, device-related thrombus was reported in 7 patients, no patients experienced pericardial effusion requiring open cardiac surgery, and there were no device embolizations. This study demonstrates the overall improved safety of the latest generation, WATCHMAN FLX device.
Some risks and complications from the WATCHMAN procedure can include:
-
The lining of the LAA is thin, so it is possible for the device to perforate the heart tissue, resulting in emergency heart surgery. This is an uncommon, but very severe complication of the procedure. In a previous study of over 38,000 patients, the risk of perforation requiring correction was 1.39%
-
Because this procedure is done under a general anesthetic, there are risks for reactions to the anesthetic medications.
-
There is a very small risk for infection at the site of the catheter insertion in the leg. Infections can delay healing time.
-
The device can dislodge from its intended position and embolize to other parts of the body. This risk is very low. Reports rates of embolization are far below 1%, usually around 0.3%
Determining Eligibility for the WATCHMAN Device
The following patients with AFib may qualify for a WATCHMAN procedure:
-
Have AFib with no heart valve issues
-
Have been recommended to start a blood-thinning medication to manage their AFib due to an elevated risk for stroke.
-
Have an appropriate rationale to seek a non-pharmacologic alternative to anticoagulation therapy. Potential rationale may include past history of major bleeding or future risk of major bleeding, to name a few.
Patients who may NOT qualify to have the WATCHMAN device implanted include:
-
Patients who are unable to tolerate short-term blood thinning medications.
-
Patients who cannot tolerate any minimally invasive procedures
-
Patients with allergies to any of the components of the implant
-
Patients with anatomically large or small LAA sites that would not fit a WATCHMAN device
Assessing Your Risk Factors
AFib patients with specific risk factors, such as an increased risk of stroke or an inability to take blood thinners, may be candidates for the WATCHMAN Device. It’s crucial to have an in-depth conversation with your doctor about your individual risk factors, as this will help determine if the WATCHMAN Device is the most appropriate treatment option for you.
Consultation with a Specialist
Consulting with a specialist is essential in determining if the WATCHMAN Device is the best treatment for you. A comprehensive evaluation of your condition, medical history, and potential risks and benefits of the implantation will be conducted during this consultation.
This tailored approach ensures that you receive the most suitable care and can make an informed decision regarding your treatment.
Learn more about WATCHMAN and find an implanting physician near you here
KardiaMobile 6-Lead Personal EKG Monitor – Six Views of The Heart – Detects AFib and Irregular Arrhythmias – Instant Results in 30 Seconds – Works with Most Smartphones – FSA/HSA Eligible
13% OffEnhancing Quality of Life with the WATCHMAN Device
The WATCHMAN Device offers a new lease on life for many AFib patients by significantly lowering their stroke risk while no longer needing strong blood thinning mediations. By closing off the LAA and preventing blood clots from entering the bloodstream, the device effectively eliminates the primary source of stroke-causing blood clots in AFib patients. The potential to discontinue strong blood thinners after the WATCHMAN Device implantation further enhances patients’ quality of life, as they no longer have to worry about the complications associated with long-term anticoagulant use.
For many patients, the WATCHMAN Device provides a sense of freedom and peace of mind, knowing that their risk of stroke has been significantly reduced. This breakthrough treatment offers AFib patients an opportunity to live a more fulfilling life without the constant fear of stroke and the burden of blood-thinning medications.
Reducing Stroke Risk
The WATCHMAN Device effectively lowers the risk of stroke in AFib patients by occluding the LAA, the primary source of stroke-causing clots. This minimally invasive treatment option has been proven to be secure and effective in clinical trials and is now widely available to patients worldwide.
Discontinuing Blood Thinners
In many cases, AFib patients can discontinue their strong blood-thinning medication intake after the WATCHMAN Device implantation, such as warfarin, eliquis, or xarelto. This is a significant advantage, as it eliminates the need for long-term anticoagulant use and its associated complications, including bleeding risks and food and drink restrictions. Preferably, patients should still take lighter blood thinners long term after watchman implant, which is typically a low dose aspirin daily.
Patients should work closely with their physician to develop an appropriate plan for tapering off blood thinners and closely monitor their condition during this transition.
Summary
The WATCHMAN Device represents a groundbreaking advancement in the treatment of atrial fibrillation. Its ability to reduce stroke risk, replace long-term strong blood thinners, and improve overall quality of life for AFib patients is truly life-changing. If you or a loved one are living with atrial fibrillation, consider discussing the WATCHMAN Device with a specialist to determine if this innovative treatment option is right for you. Take control of your health and embrace a future free from the constant worry of stroke.
Auto Amazon Links: No products found.
Frequently Asked Questions
What are the Pros and Cons of the WATCHMAN Device?
The Watchman device has the potential to reduce stroke risk and the need for blood thinners with a minimally invasive procedure, but it comes with certain risks and may not be widely available or covered by insurance.
It is important to understand the risks associated with the device and to discuss them with your doctor before deciding if it is the right choice for you. Additionally, it is important to check with your insurance provider to see if the device is covered.
Who Should Not get a WATCHMAN Device?
Patients who cannot take warfarin, aspirin, or clopidogrel, those who should not or cannot undergo heart catheterization procedures, and those with an allergy or sensitivity to nitinol (nickel and titanium) or any of the other materials in the WATCHMAN Implant should not get a WATCHMAN device.
Patients should discuss the risks and benefits of the WATCHMAN Implant with their doctor before deciding if it is the right treatment for them.
What are the Side Effects of a WATCHMAN Device?
The WATCHMAN procedure can lead to rare risks such as device embolization, stroke, infection, and heart failure. These potential side effects should be discussed with a doctor prior to proceeding with the procedure. As discussed above, the overall complication rate of a WATCHMAN procedure is low.
It is important to understand the risks associated with the WATCHMAN procedure before making a decision. Your doctor can provide more information about the potential risks and benefits of the procedure.
What Does the WATCHMAN Do?
The WATCHMAN is a device used to prevent the formation of blood clots in the left atrial appendage. It is small and parachute-shaped, similar in size to a quarter.
The device is implanted in the heart and works by trapping any clots that form in the left atrial appendage, preventing them from entering the bloodstream and potentially causing a stroke. It is safe and effective and has been studied in multiple clinical trials.
What is the WATCHMAN Device?
The WATCHMAN Device is a small, permanent heart implant designed to reduce stroke risk in individuals with atrial fibrillation (AFib), providing a valuable treatment option for those with an increased risk of stroke.
The device is designed to reduce the risk of stroke by closing off the left atrial appendage, a small pouch in the heart where blood clots can form and travel to the brain, causing a stroke. It is a minimally invasive procedure that can be performed in a single day, frequently with a same day discharge from the hospital.
Can You Get An MRI With A WATCHMAN Device?
Safety testing has indicated that the WATCHMAN device is “MRI Conditional.” Patients can safely get an MRI if they have the device implanted, but they must advise their MRI technician that they have the device before beginning the test.
Does a WATCHMAN device Stop AFib?
A WATCHMAN device is a treatment for stroke risk reduction, to allow patients to stop using strong blood thinning medications while maintaining stroke risk reduction. It does not treat symptoms of atrial fibrillation. Treatment of atrial fibrillation usually requires medical therapy, procedures like a catheter ablation, and lifestyle modifications. Read more about AFib treatment options here.
How long does the WATCHMAN Device Last?
The WATCHMAN device is designed to last an entire lifetime. It is a one-time procedure that does not need to be repeated. The watchman device should never have to be replaced.
Does Medicare Pay for the WATCHMAN Procedure?
Medicare and most private insurance companies have coverage for a WATCHMAN procedure since 2016. To qualify for coverage, a patient must be an appropriate candidate for the procedure as described above.
How Much Does the WATCHMAN Cost?
The cost of the WATCHMAN implant greatly depends on your insurance and medical coverage. Please speak with your medical professionals and medical insurance provider to learn more about the out-of-pocket costs of undergoing a WATCHMAN procedure.
A 2018 study looked at the cost effectiveness of a WATCHMAN implant procedure over a period of 10 years, when compared to 10 years of being on a blood thinner. Upfront procedure costs initially make the WATCHMAN procedure more expensive than warfarin and other blood thinners. However, over time, within 10 years, the WATCHMAN procedure delivers more quality-adjusted life years and has lower total costs when compared to the long-term costs of blood thinning medications.
The Best Atrial Fibrillation Book
Your Complete Guide To AFib: The Essential Manual For Every Patient With Atrial Fibrillation
$15.95 (as of June 1, 2025 14:05 GMT -06:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.) The A to Z guide on everything you need to know about atrial fibrillation. Written by AFib expert Dr. Percy Morales MD. Over 120 pages of essential information on medications, procedures, and lifestyles modifications for AFib. Easy to read for every patient.
Shop AFib Products on Amazon
KardiaMobile 6-Lead Personal EKG Monitor – Six Views of The Heart – Detects AFib and Irregular Arrhythmias – Instant Results in 30 Seconds – Works with Most Smartphones - FSA/HSA Eligible
13% Off
KardiaMobile 1-Lead Personal EKG Monitor – Record EKGs at Home – Detects AFib and Irregular Arrhythmias – Instant Results in 30 Seconds – Easy to Use – Works with Most Smartphones - FSA/HSA Eligible
$79.00 (as of June 1, 2025 14:05 GMT -06:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)
Apple Watch Series 9 [GPS 41mm] Smartwatch with Storm Blue Aluminum Case with Silver Sport Band M/L. Fitness Tracker, Blood Oxygen & ECG Apps, Always-On Retina Display
(as of June 1, 2025 07:09 GMT -06:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)
Fitbit Sense 2 Advanced Health and Fitness Smartwatch with Tools to Manage Stress and Sleep, ECG App, SpO2, 24/7 Heart Rate and GPS, Shadow Grey/Graphite, One Size (S & L Bands Included)
20% Off
OMRON 2-in-1 Upper Arm Blood Pressure Monitor & 1-Lead EKG Monitor - Clinically Validated Blood Pressure Arm Cuff & Machine - Use OMRON Connect App
17% Off
Samsung Galaxy Watch 6 44mm Bluetooth Smartwatch, Fitness Tracker, Personalized HR Zones, Advanced Sleep Coaching, Heart Monitor, BIA Sensor, Health Wellness Insights, Big Screen, US Version, Graphite
52% Off $329.99 (as of June 1, 2025 19:22 GMT -06:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)
Natural Rhythm Triple Calm Magnesium 150 mg - 120 Capsules – Magnesium Complex Compound Supplement with Magnesium Glycinate, Malate, and Taurate. Calming Blend for Promoting Rest and Relaxation.
$20.77 ($0.17 / Count) (as of June 1, 2025 15:43 GMT -06:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)
Pure Encapsulations Magnesium (Glycinate) - Supplement to Support Stress Relief, Sleep, Heart Health, Nerves, Muscles, and Metabolism* - with Magnesium Glycinate - 180 Capsules
$44.60 ($0.25 / Count) (as of June 1, 2025 07:10 GMT -06:00 - More infoProduct prices and availability are accurate as of the date/time indicated and are subject to change. Any price and availability information displayed on [relevant Amazon Site(s), as applicable] at the time of purchase will apply to the purchase of this product.)














![Apple Watch Series 9 [GPS 41mm] Smartwatch with Storm Blue Aluminum Case with Silver Sport Band M/L. Fitness Tracker, Blood Oxygen & ECG Apps, Always-On Retina Display #1](https://m.media-amazon.com/images/I/311xwtp4mFL._SL100_.jpg)
![Apple Watch Series 9 [GPS 41mm] Smartwatch with Storm Blue Aluminum Case with Silver Sport Band M/L. Fitness Tracker, Blood Oxygen & ECG Apps, Always-On Retina Display #2](https://m.media-amazon.com/images/I/41j+8AaUGsL._SL100_.jpg)
![Apple Watch Series 9 [GPS 41mm] Smartwatch with Storm Blue Aluminum Case with Silver Sport Band M/L. Fitness Tracker, Blood Oxygen & ECG Apps, Always-On Retina Display #3](https://m.media-amazon.com/images/I/41jIyxZitnL._SL100_.jpg)
![Apple Watch Series 9 [GPS 41mm] Smartwatch with Storm Blue Aluminum Case with Silver Sport Band M/L. Fitness Tracker, Blood Oxygen & ECG Apps, Always-On Retina Display #4](https://m.media-amazon.com/images/I/41IpNJERjCL._SL100_.jpg)
![Apple Watch Series 9 [GPS 41mm] Smartwatch with Storm Blue Aluminum Case with Silver Sport Band M/L. Fitness Tracker, Blood Oxygen & ECG Apps, Always-On Retina Display #5](https://m.media-amazon.com/images/I/31o17yhfYpL._SL100_.jpg)